Unravel the details surrounding the groundbreaking RSV vaccine tailored for pregnant individuals, shedding light on its safety considerations, potential benefits, and its place alongside other preventive measures. As the U.S. Food and Drug Administration greenlights this pioneering vaccine, discover its significance in safeguarding the youngest lives against RSV, while addressing questions about its integration with an antibody treatment.
A Dual Defense Strategy for RSV Protection
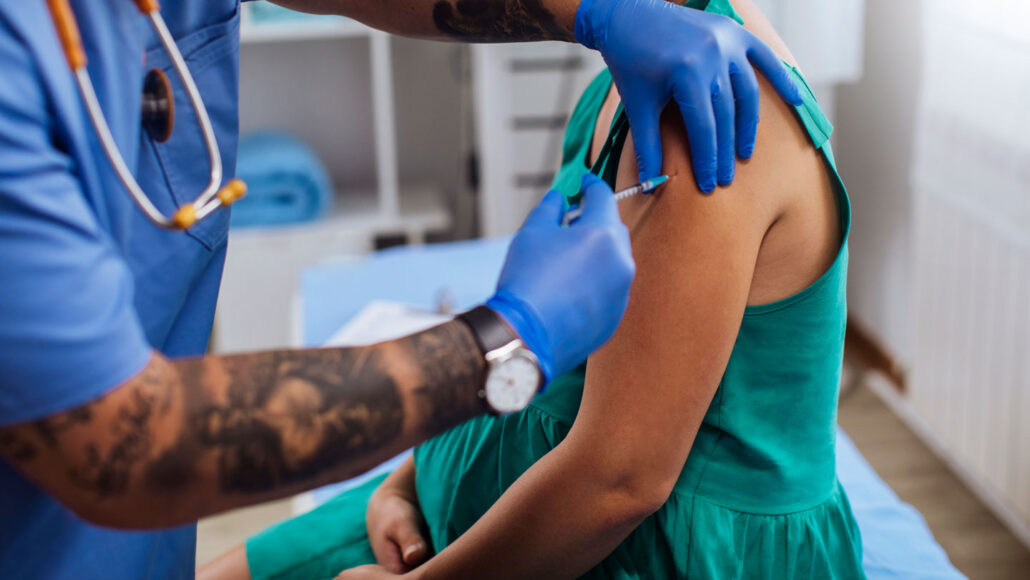
The U.S. medical landscape is poised to embrace a new defense strategy against RSV, a highly impactful respiratory syncytial virus causing respiratory illnesses, especially in infants. With the FDA’s approval on August 22, pregnant individuals have access to the first RSV vaccine. Administered between the 32nd and 36th weeks of pregnancy, this approach aims to bolster the immune defenses of both parents, ensuring that newborns are shielded during their initial months of life.
RSV poses a significant threat, holding the unenviable title of being the primary cause of infant hospitalizations in the United States. Each year, this virus sends an estimated 58,000 to 80,000 children below the age of 5 to the hospital. This innovative vaccine adds to the preventive arsenal, alongside the recently approved antibody treatment Beyfortus (nirsevimab), championing the fight against RSV.
Navigating Uncertainties in RSV Protection Strategy
The outcomes of a trial involving around 7,000 pregnant participants underscore the vaccine’s promise, revealing that fewer infants born to parents receiving the Pfizer-produced vaccine faced severe illnesses compared to those born to placebo recipients.
Despite these encouraging results, the practical utilization of the new vaccine and its compatibility with other interventions remains shrouded in uncertainty. Renowned pediatric infectious diseases physician and virologist Betsy Herold, from Albert Einstein College of Medicine and the Children’s Hospital at Montefiore in New York City, highlights the yet-to-be-explored landscape of incorporating these measures into standard health care procedures.
Navigating the CDC’s Recommendations and Unanswered Queries
While the FDA’s role includes approving drugs and vaccines, the U.S. Centers for Disease Control and Prevention (CDC) assumes the responsibility of providing recommendations for their usage. On August 3, the CDC advised administering a dose of Beyfortus to all infants under 8 months old and high-risk infants aged 8 to 19 months. The imminent decision regarding the new vaccine’s administration to pregnant individuals, and its potential amalgamation with antibody treatments, rests with the CDC.
Two pivotal considerations are poised to influence the CDC’s verdict:
1. Safety Concerns Surrounding the New RSV Vaccine Data from Pfizer’s trial raise a potential concern regarding premature birth in individuals who received the vaccine. Although this trial did not conclusively attribute early births to the vaccine due to the limited sample size, it echoes a similar situation in a clinical trial by pharmaceutical company GSK in 2022. GSK’s trial also identified a heightened risk of premature birth among vaccine recipients.
This issue featured prominently during an FDA advisory committee meeting in May, where panelists debated the vaccine’s safety profile. The panel acknowledged the vaccine’s efficacy in safeguarding infants against RSV, but divided opinions emerged about whether the potential risk of premature birth could be justified by the vaccine’s protective benefits.
2. Limitations of Relying Solely on an RSV Vaccine The vaccine’s mechanism of conferring protection by transferring antibodies via the placenta before birth exhibits limitations. Premature infants, who are inherently more susceptible to severe RSV, may not reap the full protective benefits due to their early arrival. This raises uncertainties about the extent to which premature babies could benefit from this vaccination approach.
Furthermore, the duration of vaccine-induced protection remains an enigma. While the vaccine exhibits reduced risks of severe RSV for up to six months after birth, its long-term efficacy remains uncharted territory.
Balancing Vaccine and Antibody Treatment Strategies
The impending arrival of the antibody drug Beyfortus, administered via injection, adds another layer to the preventive strategy against RSV. This drug, set to debut in the coming fall, holds particular promise for safeguarding premature and higher-risk babies. However, a significant dilemma looms: the optimal timing and prioritization of using the vaccine or the antibody treatment, or potentially both.
Both approaches have their merits. Beyfortus’ antibodies, meticulously engineered to target specific virus regions and stimulate immune responses, exhibit longevity within infants’ bodies. On the other hand, the vaccine stimulates varied immune responses against multiple virus regions. This multipronged approach could prove valuable in the face of virus mutations, akin to the adaptability required against evolving variants of the coronavirus.
While the vaccine offers promise, experts acknowledge the need for further studies to explore its connection with premature birth. The CDC’s recommendations, poised for release in the near future, hold the potential to clarify these intricate matters, offering much-needed guidance for healthcare providers and parents alike.